The exact mechanism of how radial extracorporeal shockwave therapy affects human tissue is poorly understood. However, Haupt (1997) postulated 4 reaction phases.
- Physical Phase. Extracellular cavitation, ionisation of molecules and an increase in cell membrane permeability.
- Physical-Chemical Phase. An interaction between diffusible radicles and bio-molecules released from stimulated cells.
- Chemical Phase. Intracellular reactions and molecular changes in the actual cells.
- Biological Phase. Is only established if the changes occuring in the chemical phase persist.
The effects of radial shockwave can be categorised into two major areas:
- Direct effects
- Indirect effects
Both the direct and indirect effects produce a biological response in treated tissues (Ogden, To-th-Kischkat & Schultheiss, 2001, Schmitz et al., 2013, Ueberle, 2007).
Cavitation (indirect effect, in the physical phase):
The indirect effect of cavitation is probably best understood. After the initial positive pressure wave of shockwave there is a rapid negative pressure phase (tensile phase). During this negative phase cavitation occurs. Cavitation is the formation of vapour bubbles in a liquid wherever the pressure of the liquid falls below its vapour pressure (Moholkar & Pandit, 1997) (this is what happens when you click your knuckles – the gas in the liquid implodes). This indirect effect occurs in both radial and focused shock waves (Schmitz et al., 2013, Chitnis & Cleveland, 2006, Ueberle, 2007). These vacuum bubbles induce local shear forces when collapsing at the end of the phase of negative pressure (Ogden, To´th-Kischkat & Schultheiss, 2001). This cavitation damages the affected tissues.
Capturing images of the cavitation bubbles is not easy but has been performed by Kiessling et al. (2015) and Schlaudraff et al. (2014). Both studies describe the same process which can be seen here.
Although pictures of cavitation are hard to generate radial shockwave machines have been captured creating them. An example is seen below:

Cavitation bubbles 15mm head 4bar at 10hz in degassed water.
The cavitation appears to last 1ms per shock and appears to be greater with increased shock frequency (Schlaudraff et al., 2014). Lower frequencies (1hz) show less cavitation (Schlaudraff et al., 2014) whilst low frequencies (5hz) show good cavitation (Kiessling et al., 2015) and high frequencies (15hz) show almost black out with cavitation. Of note the higher the frequency the wider Schlaudraff et al. (2014) found the cavitation field to be, but they also found the pressure field to be lower (for direct effects). Examples can be seen below:

Cavitation bubbles 15mm head 4bar 1hz in degassed water

Cavitation bubbles 15mm head 4bar 5hz in degassed water

Cavitation bubbles 15mm head 4bar 15hz in degassed water
Shockwave is thought to act on real cells in a mechanical way with three main consequences:
- Cell destruction
- Cell permeabilization
- Cell detachment
Destruction (direct and indirect effects in the physical phase):
Destruction of tissue by shockwave is still a debated topic (we know it happens as kidney stones are destroyed this way). There are 4 main ways shockwaves can destroy tissue:
Spalling/spall crack formation (direct effect in the physical phase). This is due to the generation of tensile stress caused by shockwave reflections at a pressure-release boundary (Delius, 2000, Lokhandwalla & Sturtevant, 2000, Eisenmenger, 2001, Xi & Zhong, 2001, Zhu et al., 2002). Reflected tensile waves may be focused or superimposed with the initial tensile pulse (Gracewski et al., 1993, Dahake, 1997, Xi & Zhong, 2001).
Spalling (direct effect in the physical phase) can be referred to as fragmentation. If a high velocity impact (in this case a shockwave) is applied at one side of a tissue, and can reach the other side (to a point where 2 tissues meet), then spalling starts from the free end (the impacted side where the shockwave hits). The compressive pulse is reflected from one side of the structure to the other as a tensile pulse. If the tensile pulse is higher than the ultimate tensile strength of the material a spall is formed, and the opposite side of the structure breaks off (Tan, 2008, Yankelevsky & Avnon, 1998).
Imagine a bony heel spur. The shockwave hits the spur –

If the wave is stronger than the tissue – and if it has sufficient depth to reach the opposite side of the scar tissue –
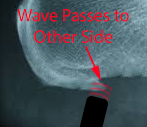
A small piece of the spur is broken off on the opposite side as the wave is reflected across the scar.

The reflected wave – if it has enough energy left – and can cross back to the other side – can now knock off another piece on the leading edge. And on and on providing there is enough energy.
A new free end is formed and the remaining original pulse is reflected from this new free end. Once again the structure breaks when the reflected tensile pulse exceeds the ultimate tensile strength of the material, and again a new free end is formed. In this way multiple spalling is generated (Tan, 2008, Yankelevsky & Avnon, 1998).
Spall crack formation can occur where the shockwave does not reach the other side of a structure.
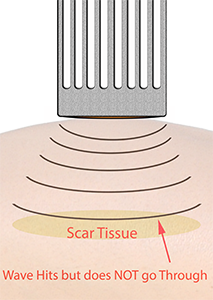
Now each impact from the shockwave generates micro-cracks in the structure.

These can then coalesce into one major crack. Spall is the process of internal failure or rupture of condensed media through the nucleation, growth and coalescence of defects, such as micro-cracks, due to stresses in excess of the tensile strength of the material (Tan, 2008, Yankelevsky & Avnon, 1998).
Damage caused by cavitation erosion (indirect effect in the physical phase)
Church (1989), Delius (2000), Lokhandwalla & Sturtevant (2000), Eisenmenger (2001), Xi & Zhong (2001), Zhu et al. (2002), Coleman et al (1987), Crum (1988), Sass et al. (1991), Zhong, Chuong & Preminger (1993), Rink et al. (1994), Delius (1997) all showed this phenomena occurs where there are transitions between tissues. In this case a piece of scar tissue lays beneath the skin. Above the scar tissue is fluid rich tissue (fat, fascia etc.). Cavitation forms above the scar (we miss the scar with our settings) and now acts on the fluids in the tissues. This process often occurs fractions of a millimetre above the scar as cavitation likes to occur on the boarder between tissues (this is because there are two tissues with different tensile strengths which shear against each other as the shockwave affects them differently).

The cavitation creates hyper-heating of fluids creating small ‘jets’ of fluid which ‘shoot off’ from the cavitation. (We have all created this process cooking when we add food to hot oil. When the water in the food hits the oil it is hyper-heated and it ‘spits’ or ‘jets’ out of the pan which hurts if it hits you).

These ‘jets’ cause damage to the tissues both directly and as a cavitation ring on the surface.

Where there are jet impacts on the tissues secondary shock wave creation can occur on a small scale during bubble collapse.
Tension (direct effect in the physical phase) induced by pressure gradients of a shock wave (Eisenmenger, 2001).
When shockwaves pass between tissues/cells of different types the waves meet different resistances. These changes impede the movement of the waves non-linearly (in other words the whole wave does not change at the same time as the tissues/cells are strange shapes not shockwave shape).

As you can see the wave and scar tissue are not the same shape.
This impeded motion creates shear forces on the boundary between the different tissues/cells.

The edges of the transition rub against each other.
These shear forces create damage at the transition destroying cells and triggering the healing process.

The destruction is on both sides of the transition.
Squeezing (direct effect in the physical phase) of the tissue due to circumferential pressure (Eisenmenger, 2001).
Sometimes the wave will be of sufficient size (this happens at the cellular level all the time) to envelop the whole of the structure (in this case a small piece of scar tissue).

The scar tissue fits between the wave boundaries.
Now the tissue is squeezed and pulled out of shape.

This pressure can pull the tissue to any shape.
Often the pulling or squeezing effect will shatter the structure or break it apart.

Cell permeabilization and molecular delivery (direct effect in the physical phase)
All cells are enclosed in plasma membranes only a few nanometers thick and the lipid molecules that make up these membranes are able to be displaced. Besides keeping the cell together, the plasma membrane also controls the transport of fluids and molecules between the extracellular (outside environment) and intracellular medium (what’s inside the cell). The control of molecular transport across the membrane is crucial for cell survival. However, in medicine these controls can be circumvented. This allows us to insert normally membrane impermeable molecules into the cell. Such a procedure is called molecular or drug delivery (known in ultrasound as iontophoresis). A special kind of delivery into the cell is the insertion of genes. If the DNA-sequence reaches the nucleus it can be expressed by the cell into proteins. Currently there exist several different approaches to facilitate gene delivery (Miller, 1992).

A method for creating cell permeabilization and drug delivery which is based on laser therapy is called optoporation (Soughayer et al., 2000). A laser with sufficient intensity (at the focal spot) to exceed the breakdown limit of the liquid in the plasma is focused at the cell. A shock wave is then emitted and a vapour bubble originates at the focal spot. Due to the outward acceleration of the surrounding fluid the bubble first expands, then it contracts and emits a second shock wave during the final stage of the collapse. Cells in the vicinity of an optical breakdown can be transiently permeabilized. The permeabilization is probably caused by shear stress (Doukas & Flotte, 1996, Lee et al., 1996, Lee et al., 1997) which are either generated by the outgoing shock waves or by a strong flow field during the rapid bubble dynamics.
It is possible that in vivo application by acoustic means could change the permeabilization of cell membranes, commonly called sonoporation. Gambihler et al. (1994) have shown that application of shock waves can facilitate the uptake of usually membrane impermeable molecules in vitro. Since then many studies have been performed to assess membrane proration and molecular delivery after shock wave exposure in vitro (Bao, Thrall & Miller, 1997, Delius et al, 1995, Greenleaf et al., 1998, Brayman et al., 1999, Huber et al., 1999, Miller, Bao & Morris, 1999, Qian, Sagers & Pitt, 1999, Ward, Wu & Chiu, 1999, 2000, Zhong et al., 1999, Koch et al., 2000, Kodama, Hamblin & Doukas, 2000, Miller & Quddus, 2000, Guzman et al, 2001, Tschoep et al., 2001, Ross et al., 2002, Wu, Ross & Chiu, 2002) and in vivo (Miller et al., 1999, Miller & Quddus, 2000, Miller & Gies, 2000, Miller, 2000, Teslenko et al., 2002). Although intensive research in quantifying sonoporation has been performed in the last years, the mechanisms which are responsible for the permeabilization of cells during shock wave applications are not yet fully understood. However, similar to optoporation they are probably based on direct interactions between the pressure wave and the cell or on the generation of cavitation bubbles.
Cell Adhesion (direct effect in the physical-chemical phase)
Cell adhesion is a critical part of life on earth and is particularly important in living tissues as without it cells would not remain together and we could not be multi-cellular organisms. Adhesion is essential to the cohesion of tissue, cell migration, the formation of organs, wound healing, immune response after inflammation and pathological tumour invasion (Alberts et al., 1994, Zhu, Bao & Wang, 2000). There is significant diversity of cell adhesion in each of these processes so it would seem reasonable that the adhesion process is variable for different cell types or even the same cells under different conditions. The whole process is made more complicated when we consider that cells don’t just adhere to each other they can also adhere to the extracellular matrix. You can distinguish between cell-cell adhesion and the attachment of cells to the extracellular matrix, but in both cases the attachment of cells is mediated by linker proteins (Alberts et al., 1994). An important family of transmembrane linker proteins for cell-cell adhesion are the cadherins. Members of this family usually act as a bridge between two cells but attach at each end differently (they display homophilic binding, where the same type of molecule acts as receptor and ligand on two adjacent cell surfaces). Cells are supposed to form robust attachments at cell-cell or cell-matrix adhesion sites – it is not suffcient for adhesion molecules to simply connect to the plasma membrane of the cell. In fact, to generate a firm junction the cell adhesion molecules must be linked to the stable structure of the cytoskeleton inside the cell. This is achieved by intracellular attachment proteins, which connect the intracellular part of transmembrane linker proteins to either actin or intermediate filaments of the cytoskeleton.

Often several adhesion molecules work together to create focal contact junctions at specific spots. Through the junctional attachment sites forces can be mediated from cells to an extracellular matrix and vice versa. For example, in medicine fibroblast cells generate tension on the intracellular filamentous network, this has been suggested to be by the action of molecular motors, this in turn is transmitted to the substrate (Zhu, Bao & Wang, 2000). Conversely, the extracellular matrix can influence the arrangement of the cytoskeleton affecting cell shape. Cell adhesion molecules do not only act as mechanical connectors. In red blood cells integrins have to be activated before they can mediate cell adhesion (without that we wouldn’t clot). There are also force-transmitting cell adhesion molecules (like integrins) which act as sensors, and send signals about external mechanical loads to the cell. This allows the cell to change (normally through gene expression) which in turn can result in cell growth and again further cell adhesion (imagine this is how tendons get stronger in relation to mechanical stress at the cellular level).
Cells demonstrating good adhesion display typical spreading (as seen below).

When adhesion molecules are inhibited, the cells detach and adopt a round shape (shown below).

With no adhesions the cells are no longer pulled into different shapes and revert to their natural round shape (they ’round up’). The cells are then depolymerized, depriving adhesion molecules of their intracellular anchors (Alberts et al., 1994).
Shockwave shear forces creating shear flow along the substrate can cause cell debonding. For a given cell, detachment occurs if the shear stress reaches a certain threshold. The threshold probably depends on the contact surface and the number and type of adhesion bonds.

Decave et al. (2002) describe the kinetics of shear flow induced detachment in a peeling model where adhesive bonds break progressively with time, starting at the edge of the cell facing the flow. This velcro-like detachment procedure allows for cell detachment at relatively low shear stresses compared to instantaneous whole cell detachment. Violent shear flow however, may cause rapid cell detachment and hence cellular damage.
Fibroblasts (direct effect in the physical phase and indirect effect in the chemical phase)
Radial shockwave has two main effects on fibrocytes or fibroblasts:
- Mechanical effects
- Chemical effects
Fibrocytes are found in the extracellular matrix. They normally sit in a passive state until needed when they will change shape and become fibroblasts (or basic fibroblast growth factor (bFGF) molecules). ‘Fibroblast’ is actually a catch all term used for a variety of connective cell types (Frairia & Berta, 2011). Fibroblasts are found extensively throughout connective tissue such as tendon, ligament, skin and nerves, amongst other structures, and play a role in the healing process of these structures. Fibroblasts can even turn into a type of epithelia by undergoing a mesenchymal to epithelial transition (MET) and organizing into a condensed, polarized, laterally connected true epithelial sheet sealing a wound.
bFGF
It appears there are many functional differences between fibroblasts from each of the different parts of the body which reflect the different functions for each organ (Ross, Romrell & Kaye, 1995, Wang et al., 2007). Interestingly, there are also variations among the fibroblasts within each site (Frairia & Berta, 2011). Even within one structure there may be fibroblasts with very different biochemical characteristics (seen in the lungs and skin) (Frairia & Berta, 2011). Fibroblasts often vary in their morphology, proliferation rate, surface markers, protein synthesis or response to a given stimulus (these differences are referred to as fibroblast heterogeneity) (Jelaska, Strehlow & Korn, 2000).
Fibroblasts play a crucial role in the human body by synthesizing and organizing connective tissue components. They basically make the bits we need to repair and grow connective tissue. They do this as they secrete the extracellular matrix precursors for healing. Fibroblasts decide the balance in the extracellular matrix between having a lot of spare bits and pieces (most notably collagen fibres, proteoglycans, noncollagen glycoproteins and elastin) and not so many (Benjamin & Ralphs, 2000). They change this balance in response to many different stimuli including cytokines and growth factors (chemical effects affected by shockwave) and mechanical stimuli of different types (including shockwaves) (Frairia & Berta, 2011).
How do they do this? In response to injury (inflammatory stimuli), a fibrocyte undergoes a physical change including developing a large rough endoplasmic reticulum and changing shape. They move from the edge of damaged tissues into the zone of injury, once there they start to produce more fibroblasts, and accelerate destruction of damaged tissue. They replace damaged extracellular matrix and they also create additional autocrine and paracrine mediators including IL-1, IL-6, IL-8, TGF-β, prostaglandins and nitric oxide (NO) (Long & Brown, 2002).

Fibrillin glycoprotein molecule
Fibroblasts are mechanoresponsive. This means they react to external stimulus that mechanically hits them. Shockwaves do exactly this through mechanotransduction. This is the cellular process by which physical forces are converted into biochemical signals (Huang, Kamm & Lee, 2004). Shock waves create physical force in a variety of ways such as through substrate stretching or through movement of fluid (see squeezing and cavitation jets above). Membrane proteins such as ion channels, integrins and associated cytoplasmic complexes are all mechanoreceptive and they are all switched on by physical stimuli from shockwave. The cells stimulated by physical forces interact with the cells around them. There is a transmission through protein-protein interactions via transmembrane proteins (Vogel, 2006).
Shockwave can even influence conformational changes of cell membrane proteins making unmasked cryptic binding or phosphorilation sites, or even regions that display enzymatic activity (Bustamente et al, 2004, Tamada, Sheetz & Sawada, 2004).

The size of focal contact points and the following signalling activity is force dependent (Galbraith, Yamada & Sheetz, 2002). The following intracellular signalling affects gene expression with alteration of binding properties and/or of enzymatic functions (Shyy & Chien, 1997).
The integrins, which are responsible for connecting cells to the extracellular matrix, can trigger signals in response to shockwave (Shyy & Chien, 1997, Galbraith & Sheetz, 1998, Zhou et al, 2004). They are mechanoreceptors and literally pull on specific adhesion proteins which are sensitive to tension which in turn activate intracellular signals (Regent et al, 2011). Thus, integrins change mechanical stress into chemical signals.

Shockwave probably accelerates integrin activation, both by extracellular and intracellular forces, and induces protein recruitment through protein stretching (Puklin-Faucher & Sheetz, 2009).
Frairia and Berta (2011) tell us that connective tissue cells are able to distinguish between various modes of mechanical stress: compressive e.g. in cartilage (Guilak et al, 2000), tensile e.g. in tendons (MacKenna, Summerour & Villarreal, 2000) and shear e.g. in blood vessel walls (Davies et al, 1997). Integrins are said to be the mechanoreceptors in a wide range of cells including myocytes, fibroblasts, endothelial cells, chondrocytes and bone cells (Rubin, Rubin & Jacobs, 2006).
Activation/recruitment of mesenchymal stem cells (Wang et al., 2004 and Chen et al. 2004). Mesenchymal stem cells appear to react to shockwave just like fibroblasts. When mechanically stimulated, mesenchymal stem cells adjacent to a segmental defect are subject to three consecutive events after shockwave therapy:
- intensive recruitment and proliferation
- chondrogenic differentiation
- osteogenic differentiation
ESW treatment has been shown to promote bone regeneration by stimulation of recruitment and differentiation of mesenchymal stem cells (Chen et al., 2004).
Growth Factors (direct effect in the biological phase)
Growth factors release after mechanical stimulation is a chemical effect of shockwave. Cells change in relation to stress, in this case mechanical stimulation through the release of growth factors, and this is an initial step in the regeneration of new supporting connective tissue (Frairia & Berta, 2011, Rubin, Rubin & Jacobs, 2006). Studies have indicated that mechanical loading (like shockwave) increases the expression of several growth factors and cytokines, such as Insulin-like Growth Factor 1 (IGF-1), Transforming Growth Factor-β1 (TGF-β1), and Interleukin-6 (IL-6) (Heinemeier et al, 2003). Cyclic stretching of fibroblasts (as seen in squeezing above) is said to modulate secretion patterns of growth factors (Skutek et al, 2001) with an increased release of Fibroblast Growth Factor-2 (FGF-2), TGF-β1, and Platelet Derived Growth Factor (PGDF). In vitro as well as in vivo experiments have shown an increased expression of TGF-β in response to mechanical stimuli in a number of cell and tissue types. Going further, mechanically induced type I collagen expression in ligament fibroblasts is directly dependent on TGF-β activity (Heinemeier et al, 2003).

TGF1
Loading stimulated type I and/or type III collagen development appears to depend directly on TGF-β1 activity in human ligament and patella tendon fibroblasts (Yang, Crawford & Wang, 2004). Several studies point to TGF-β1 as an essential mediator of mechanically induced collagen synthesis in a variety of cell types. In addition to TGF-β1, Connective Tissue Growth Factor (CTGF) could be a link between loading and collagen synthesis (Heinemeier et al, 2007, Heinemeier et al, 2009). TGF-β1 is known as a great starter of collagen growth, and it’s presence in response to loading may well be important for mediating mechanically induced type I and/or type III collagen expression in tendon and muscle tissue (Yang, Crawford & Wang, 2004, Heinemeier et al, 2007 Heinemeier et al, 2009). While collagen type I is the main component of collagen fibers, collagen type III has been shown to be important in the regulation of initial fibril assembly and thus at the early stages of injury repair (Birk & Mayne, 1997). This important process of creating new collagen after injury is a chemically mediated role of shockwave mechanical stimulation.

Collagen
But shockwave can destroy the same cells we now say we are treating. How is this possible?
Berta et al. (2009) treated normal fibroblasts in suspension with low to medium energy shock waves and evaluated fibroblast viability, the growth rate and pattern, and gene expression for TGF-β1 and collagen types I and III – the main factors involved in the repair process.
Low to medium energy shockwave treatment induced much less cell destruction and there was a better subsequent stimulation of cell proliferation. Wang et al. (2001, 2002) and Martini et al. (2003) found the same thing. Whether fibroblasts lived or died was influenced most by the number of shots much more than by energy level. There was also evidence of a suitable energy/shot number ratio to have a minor cytocidal effect. Shock waves had a dose-dependent destructive effect on cells in suspension, as well as having a dose-dependent stimulatory effect on cell proliferation. In addition, a significant increase in proliferation rate was observed with respect to the unshocked cells (this is probably because of the interconnections through the integrins).
A critical increase in cell growth was observed from the sixth to the twelfth day of the proliferation curve (Frairia & Berta, 2011). This could affect the number of sessions you want to give. i.e. to destroy tissue treat every 6 days or less, to make repairs/new tissue treat every 12+ days. Bear in mind that if the goal of shockwave treatment of tendon lesions is to promote and improve the repair process authors have concluded that treatment at the 0.22 mJ/mm2 energy level with 1,000 impulses appears to be the best in terms of fibroblast viability fitting growth dynamics. TGF-β1 and mRNA showed higher values in treated fibroblasts than in untreated fibroblasts for day 6 (p = 0.02) and day 9 (p = 0.02) respectively. Elevated expression of mRNA was observed for collagen types I and III (sixth day for collagen type I and ninth day for collagen type III). In both cases, shockwave treatment enhanced expression of the genes encoding collagen types I and III. The timing of increase of mRNA expression for TGF-β1 and for collagen types I and III mirrors that of collagen types I and III in repairing processes and confirms that TGF-β1 is involved in differentiation of fibroblasts (Chao et al, 2008, Wang et al, 2002).
If shockwave can release growth factors in tendon then can it do it in similar tissues like bone? Hausdorf et al. (2011) looked at shockwave therapy to trigger the release of bone growth factors (namely TGF-β1 and FGF-2) from cells like fibroblasts and osteoblasts (both of which are essential in a non-union treatment). While Transforming Growth Factor-β1 (TGF-β1) is responsible for multiple reactions in tissue growth and is not only produced in osteoblasts and fibroblasts (Lieberman, Daluiski & Einhorn, 2002), Fibroblast Growth Factor-2 (FGF-2) is actually a more selective bone growth factor. Fibroblast and osteoblast production of TGF-β1 and of FGF-2, predominant elements in the osteoneogenesis cascade, were shown to be increased after ESW treatment.
Oxidative stress (direct effect in the chemical phase leading to biological results).
One of the major direct effects of shockwave on cells in the areas that are not destroyed (or are treated at low shockwave levels) is oxidative stress (Deiling et al, 2012). Oxidative stress is created when there are more reactive oxygen species (ROS) in an area. ROS are chemically reactive molecules containing oxygen e.g. oxygen ions and peroxides. ROS are formed as a natural byproduct of the normal metabolism of oxygen and have important roles in cell signalling and homeostasis (Devasagayam et al. 2004). However, during times of environmental stress (e.g., mechanical stress from shockwaves), ROS levels can increase dramatically (Devasagayam et al. 2004). One of the consequences of increased oxidative stress and hence ROS is the production of Nitrous Oxide (NO).

ROS
Nitric Oxide (indirect effect in the chemical phase leading to biological effects)
A major chemical effect of shockwave therapy appears to be rapid elevation of nitric oxide (NO) levels systemically (Gotte et al., 2002). Shockwave does this by altering a chemical balance (increases ROS). Under normal conditions this change in the chemical balance stimulates the un-coupling of Endothelial nitrous oxide sulfate (eNOS) into nitrous oxide (NO). This only happens though if the presence of tetrahydrobiopterin (BH4) and dihydrobieopterin (BH2) are kept at a specific ratio (both of these molecules can couple to eNOS and the affinity is equal) which modulates eNOS uncoupling to NO. Mechanical stress affects BH4 and BH2 as well and can alter coupling to eNOS. BH4 is more susceptible than BH2 which can alter the chemical balance. So if shockwave is done to too high a level then BH2 can produce superoxide (SO) instead of NO. This SO is then later converted to H2O2 by superoxide dismutase (this can be harmful to cells). Therefore the ratio of BH4 and BH2 coupling to eNOS will determine whether eNOS principally produces NO or SO (Deiling et al, 2012).
NO is an important cellular signalling molecule. It has several main effects:
- Mediation of bone healing through increases in systemic osteogenic factors in non-union of long bone (Maier et al, 2003, Wang et al, 2009) and as the mediator in callus formation in fracture healing after mechanical stimulation (Diwan et al, 2000).
- Mediation of angiogenesis (along with Vascular Endothelial Growth Factor [VEGF]) (Babaei & Stewart, 2002, Spyridopoulos et al, 2002)
- Improved blood flow locally as shockwave induced NO is derived from eNOS (Frairia & Berta, 2011).
- Reduction of inflammation (Mariotto et al., 2009)

NO
Bone healing (direct effect in the physical and chemical phases)
Yin et al. (2011) showed that extracorporeal shockwave enhances osteogenesis gene expression in bone marrow stromal cells from hips even with osteonecrosis through the NO-mediated pathway. This showed NO stimulation can lead to bone healing even in the presence of severe bone pathology.

Femur Fracture
Induction of bone fractures (direct effect in the physical phase).
Delius et al. (1995) showed shockwaves could lead to bone fracture through spalling as explained above.
Blood supply (direct effect in the physical phase and chemical phase leading to changes in the biological phase)
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels. Wang et al. (2003) showed that shockwave therapy induces the critical factors required for angiogenesis
- Endothelial (these cells need to be present in high numbers to allow fibroblast growth factors (fgf1 and 2) to organise them into tube like structures).
- VEGF (like fgf1 and 2). VEGF can create tube like structures but more importantly causes a massive signalling cascade in endothelial cells increasing the number of capillaries in a given area.

Improved blood flow: Nitric oxide synthase (eNOS) also known as nitric oxide synthase 3 (NOS3). eNos creates NO through calcium-calmodulin controlled isoenzymes eNOS (endothelial NOS). eNos is a vasodilator as it regulates smooth muscle tone. This means you get more blood flow to an area under shockwave as well as more capillary ingrowth (Frairia & Berta, 2011).
Reduction of inflammation. Mariotto et al. (2009) clinically observed the anti-inflammatory action of shock waves and surmised it may be mediated by a shockwave induced increase in NO production.
Shockwave therapy appears to increase angiogenesis even in the first week, with cell proliferations and formation of neovessels in approximately four weeks. The neovascularization may lead to the improvement of blood supply and play a role in tissue regeneration. Along with increases in eNOS and VEGF this neovascularization may play a role in pain relief and the repair of chronically inflamed tissues (Frairia & Berta, 2011).
Neural effects (direct effect in the physical phase and chemical phase leading to effects in the biological phase)
Shockwave can influence nerves at both the macro and micro levels. The influences create several effects which may or may not be positive in the treatment of patients. These include:
- Improved blood flow (Zimmermann et al., 2009, Frairia & Berta, 2011)
- Reducing muscle tension/stiffness (Zimmermann et al., 2009)
- Reduction of pain through various mechanisms (Jeon et al., 2012)
- Generation of action potentials (Schelling et al. 1994)

Zimmermann et al. (2009) found that shockwave improved blood circulation (in capillary blood vessels) and reduced both the tension and stiffness of muscles.
Reduction of pain (direct and indirect effects both mechanical and chemical leading to biological effects)
The main reasons for shockwave affecting pain in the current literature are:
- Shock waves stimulate the nociceptors to fire high-frequeny nerve impulses (hyperstimulation). Propagation of nerve impulses is blocked according to the gate-control theory (Zimmerman et al. 2009, Hausdorf et al. 2008).
- Shock waves distort parts of, or the whole of, the cell membrane (see cell permiabilization). The nociceptors cannot build up a generator potential; thus pain sensation is avoided. (Bao, Thrall & Miller, 1997, Delius et al, 1995, Greenleaf et al., 1998, Brayman et al., 1999, Huber et al., 1999, Miller, Bao & Morris, 1999, Qian, Sagers & Pitt, 1999, Ward, Wu & Chiu, 1999, 2000, Zhong et al., 1999, Koch et al., 2000, Kodama, Hamblin & Doukas, 2000, Miller & Quddus, 2000, Guzman et al, 2001, Tschoep et al., 2001, Ross et al., 2002, Wu, Ross & Chiu, 2002)
- Shock waves change the chemical environment of the cell membranes by generating free radicals (see oxidative stress), which in turn result in pain-inhibiting chemicals in the vicinity of the cells. (Deiling et al, 2012).
- Substance P release initially after shock wave application (Holzer, 1991) then diminished number of neurons immunoreactive for substance P in dorsal root ganglia (Keen et al., 1982).
- Decreases calcitonin gene-related peptide (CGRP) immunoreactivity in dorsal root ganglion neurons (Kress et al., 1999, Takahashi et al., 2003).
- Selective loss of unmyelinated nerve fibres after extracorporeal shockwave application to the musculoskeletal system (Krischek et al., 1999).
Zimmerman et al. (2009) found shockwave reduced pain by interfering in the process of flow of excessive stimulation of nociceptors and stimulation of nerves. Hausdorf et al. (2008) found that shockwave could lead to reduction in neural activity through the selective destruction of non-myelinated nerve fibres.

Maier et al. (2003) showed that the initial burst of pain (for the first 6 hours after shockwave application) came from an increase of release of substance P (through C fibre and A-delta fibre depolarisation) and the subsequent inflammatory response from that release. Periosteum is known to contain substance P immunoreactive nerve fibres, which are also found in bone marrow, synovial membrane and soft tissues adjacent to bone (Bjurholm et al., 1988). When mechanically stimulated they release substance P (Maier et al. 2003). However this initial release is followed by a subsequent decrease in levels of substance P (and hence inflammation) at the 24 hour point as the nerve degenerates (Maier et al. 2003). This reduction in substance P release lasts for over 6 weeks (Maier et al. 2003) and may go on as long as 2 years (Maier et al. 2003). Hausdorf et al. (2008) found that application of extracorporeal shockwaves to distal nerves lead to reduced concentration of substance P in the shockwaves ‘focal zone’. (Substance P appears to have a role in potentiating both excitatory and inhibitory inputs to spinal nociceptive neurons, in effect sensitizing the neurons to any synaptic input). They also found that the application of extracorporeal shockwaves caused a significant decrease in the number of neurons immunoreactive for substance P in the treated side compared with the untreated side, without affecting the total number of neurons within this dorsal root ganglion. This study suggested that shockwave was effective at reducing pain whilst not affecting the other nerves within the bundles as significantly. Jeon et al. (2012) surmised that shockwave improved pain by inhibiting excessive excitement of nerve cells due to reduction in the synthesis of substance P at the dorsal root ganglia, while at the same time preventing central sensitization by inhibiting nociceptors of peripheral muscles.
Decreases in levels of the neuropeptide calcitonin gene-related peptide (CGRP) have been shown in studies (Kress et al., 1999, Takahashi et al., 2003). CGRP is expressed by nociceptors and plays a role in the sensation of joint pain (He et al. 1990). CGRP has been shown by immunohistochemistry to be expressed in nerve fibres supplying the rat knee at both the level of the dorsal root ganglion (DRG) (Fernihough et al. 2005) and locally in the knee (Schwab, Bilgicyildirim & Funk, 1997). Increased synthesis of CGRP peptides in DRG may play a role in the pathogenesis of chronic arthritis (Staton et al. 2007). A significant decrease in the number of CGRP-immunoreactive DRG neurons was found after shockwave treatment that was also correlated with behavioural improvement (Ochiai et al, 2007). However, the effects of CGRP only lasted for a maximum of 28 days.
Schelling et al. (1994) found that shockwave therapy can directly generate action potentials in nerves. They found that the high pressures in the focal area were directly responsible for the shock wave effect, indicating that the steep pressure gradient of the shock pulse resulted directly in axonal depolarization. Direct depolarization was also suggested from a previous experiment in which nerves were exposed to shock waves generated by mechanical impact of a steel ball on a plate (Wehner and Sellier, 1981). A similar direct effect has been described with mechanical stimuli in the microsecond range. The response to this stimulation has been explained by mechanical compression leading to a distortion of the axonal contents, strain of the membrane and a resulting increase in permeability with depolarization (Julian and Goldman, 1962, Yamada and Sakada, 1961). Schelling et al. (1994) postulated that this was the mechanism through which shockwave could be used to re-establish nerve signals in nerves that were not transmitting correctly. Unfortunately, this is also the mechanism through which shockwave therapy can stop the heart (or cause arrhythmias) and because we do not use radial shockwave in conjunction with an ECG (which would allow shocks to only be delivered at the absolute refractory period of the ventricle) radial shockwave should not be applied near the heart.
Inflammation (direct effect in the chemical phase leading to effects in the biological phase)
Inflammation can be classified as either acute or chronic. Acute inflammation is associated with the increased movement of plasma and leukocytes (especially granulocytes) from the blood into the injured tissues. A series of biochemical events propagates and matures the inflammatory response, involving the local vascular system, the immune system and various cells within the injured tissue. Shockwave can interfere with these processes. Prolonged inflammation (chronic inflammation) leads to a progressive shift in the type of cells present at the site of inflammation and is characterized by simultaneous destruction and healing of the tissue from the inflammatory process.
Shockwave can interfere with several of the precursors to inflammation affecting the cycle and hence the inflammation itself.
Acute inflammation:
Shockwave interferes with the serum levels of substance P and CGRP (see above). Both of these substances act on target cells in the periphery such as mast cells, immune cells and vascular smooth muscle cells, producing heat, swelling, redness and improved healing rate (Zimmerman et al, 2009, Maier et al., 2003).
Chronic Inflammation:
The inflammatory response must be actively terminated when no longer needed to prevent unnecessary damage to tissues. Failure to do so results in chronic inflammation and cellular destruction (Cotran et al., 1999). Resolution of inflammation occurs by different mechanisms in different tissues. However, the activity of mast cells (cells involved in the inflammatory process) may be increased by shockwave. Mast cells play a key role in the inflammatory process. When activated, a mast cell rapidly releases it’s characteristic granules and various hormonal mediators into the interstitium. Mast cells can be stimulated to degranulate by mechanical stress (Prussin & Metcalfe, 2003).

Mast cell stimulation is followed by the release of molecules into the extracellular environment which include:
Pre-formed mediators (from the granules):
- serine proteases, such as tryptase
- histamine (2-5 pg/cell)
- serotonin
- proteoglycans, mainly heparin (active as an anti-coagulant)
Newly formed lipid mediators (eicosanoids):
- thromboxane
- prostaglandin D2
- leukotriene C4
- platelet-activating factor
- cytokines
- eosinophil chemotactic factor
These are the key chemicals that start inflammation. The releasing of pro-inflammatory compounds can help to restore the normal healing and regenerative processes. This allows the process of repair to finish and then the inflammation can stop as it should have done.